GRADE
9
Science
This course enables students to develop their understanding of basic concepts in biology, chemistry, Earth and space science, and physics, and to relate science to technology, society, and the environment. Throughout the course, students will develop their skills in the processes of scientific investigation. Students will acquire an understanding of scientific theories and conduct investigations related to sustainable ecosystems; atomic and molecular structures and the properties of elements and compounds; the study of the universe and its properties and components; and the principles of electricity. Prerequisite: Science 8
TABLE OF CONTENTS
Biology: Sustainable Ecosystems
Plant Systems: Energy Production
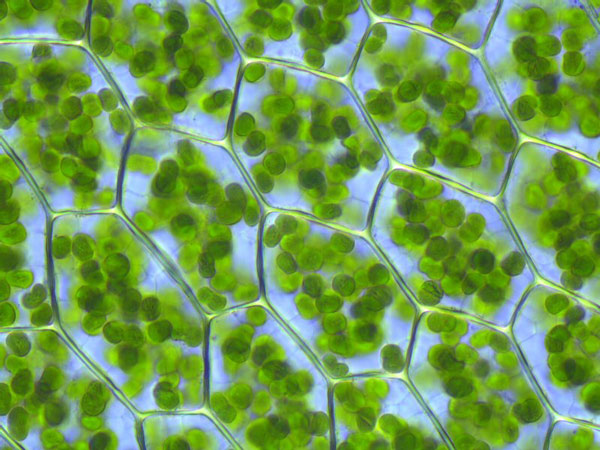
The process in which plants convert light energy into chemical energy is called
Solution
Photosynthesis is how plants store their energy.
 |
Plant Systems: Energy Production
Which of the following gases is a by-product of cellular respiration in plants? Solution
 |
Cellular respiration should not be confused with photosynthesis! Yes plants can respire also, like we humans can.
Plant Systems: Energy Production
In plants, which of the following occurs during both the day and at night? Solution
 |
Photosynthesis cannot occur at night, leaving only cellular respiration.
Plant Systems: Energy Production
What molecule does a plant use to store its energy?
Solution
Photosynthesis creates the sugar out of sunlight, water, and carbon dioxide.
Soil Composition
Leaching of organic and inorganic fertilizers or pesticides into water systems can damage the watersheds. How do farmers minimize this risk?
Solution

Legumes contain natural fertilizers, like nitrogen.
Soil Quality
Eutrophication is the when runoff leaks high amounts of nutrients into the aquatic ecosystem, causing an increase in the amount of carbon dioxide in the water.
Solution
Runoff like sewage or fertilizers add unusually high levels of nutrients to the water. The most significant impact - rather than growth of algae - is the decrease in oxygen dissolved in the water. This is due to several factors including the decomposition of high amounts of biomass.
Characteristics of Ecosystems
Within the terrestrial ecosystem, trees are considered to be
Solution

Biotic
Spheres of the Earth
The water vapour in the clouds in the air is considered to be within the: Solution
 |
The hydrosphere contains all the water, including water when it is in the atmosphere as water vapour, rain, hail...
Ecology
Grass is a(n): Solution
 |
Autotrophs store energy by making nutritional organic substances like sugars.
Biomass
The second level on the Biomass pyramid contains the
Solution
Biomass
The trophic level with the largest biomass is Tertiary consumers.
Solution
The trophic level with the largest biomass is Primary producers.
E.g.) Plants, algae, trees, grass, etc...
E.g.) Plants, algae, trees, grass, etc...
Biosystems
The process of building up toxins in larger animals is known as
Solution
Bioaccumulation
In which of the following species would the highest concentration of fat-soluble toxins be found?
Solution
Highest in the tertiary consumers at the top of the food web. The species at the top of the food web amongst these given examples is the bear.
Biosystems
A dramatic increase in the number of a certain species will:
Solution
While this has the chance of changing the sustainability of the ecosystem, it does not necessarily mean the system is ruined. Sometimes this is used intentionally to help restore a balance. Predators have more food and eat more, thriving and increasing the number of predators. Carrying capacity: the competition amongst the increased species' population will further reduce their numbers because of the limited nutrient resources.
Biosystems
In a forest ecosystem foxes are the predators of rats, and rats prey on smaller mice. If a municipality with unusually high numbers of rats were to collect them all and dump them in the forest outside of the city what would likely happen?
Solution

Many different things could happen, one of which could be an increase in the consumers as the food supply increases. (An other thing that could happen is the competition of the food supply between the rats, leading to reduction.)
Invasive Species
Zebra mussels and the goby fish are two examples of invasive species that have
Solution
These species have out-competed native species and this has caused a reduction in diversity.
Threats to Sustainability
As a researcher, what's one way to verify that overhunting or overfishing is actually happening?
Solution
These methods are currently used to monitor the populations of many different at-risk species.
Human Activity and Ecosystems
A rapidly-growing city requires a new energy source to provide power to the increasing number of residents. A nearby mass of water would provide an effective location for a hydroelectric dam, which creates power from the movement of flowing water. Describe how creating a massive artificial lake might affect the ecosystem. [2]
Solution
 Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Flooding upstream. (Maybe same amount of water downstream) although if diverted then lack of water would cause animals to move to another area with water. Animals lost habitat upstream, must move...
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Flooding upstream. (Maybe same amount of water downstream) although if diverted then lack of water would cause animals to move to another area with water. Animals lost habitat upstream, must move...
(Answers may vary... Add more possible answers...)
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
(Answers may vary... Add more possible answers...)
Chemistry: Atoms, Elements, Compounds
Mixtures
Mixtures and Solutions
There are two types of mixtures, mechanical mixtures and solutions. Which of the following is a solution?
Solution
Video
Can you visually distinguish the particles as a heterogeneous mixture? If yes, then it is a mechanical mixture. If no, then it is a homogenous (solution) mixture.
A mechanical mixture contains more than one type of visible particles like pasta sauce, jellybean mixtures, cereal with milk, and the pulp in orange juice. Mechanical mixtures are heterogeneous mixtures.
A solution contains more than one type of particle that are not visible or distinguishable, like the different gases in air (nitrogen, oxygen, argon, carbon dioxide...) Solutions are a homogeneous mixture.
A mechanical mixture contains more than one type of visible particles like pasta sauce, jellybean mixtures, cereal with milk, and the pulp in orange juice. Mechanical mixtures are heterogeneous mixtures.
A solution contains more than one type of particle that are not visible or distinguishable, like the different gases in air (nitrogen, oxygen, argon, carbon dioxide...) Solutions are a homogeneous mixture.
Provide an example of a typical solution that consists of ions. [1]
Solution
Video
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Juices, sports drinks (like Gatorade, etc) contain ions like sodium Na+, calcium Ca2+, ...
(Answers may vary...)
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
(Answers may vary...)
Provide an example of a solution that consists of a pure liquid mixed with another pure liquid at room temperature.
Solution
This is a bit difficult because most liquids students will say are unpure, aqueous solutions like juices, milks, etc... It is easier to understand a solution between one liquid (water) and any aqueous stuff, like the things mentioned previously.
Pure liquids don't have anything dissolved in them, for example:
- Water
- Ethanol
- Mercury
- Hexane hydrocarbon
- Benzene hydrocarbon
Accept any mixture of pure elements or compounds that are not visiblly distinguishable...
(Note that students do not yet understand polarity, so this could be tricky)
Pure liquids don't have anything dissolved in them, for example:
- Water
- Ethanol
- Mercury
- Hexane hydrocarbon
- Benzene hydrocarbon
(Note that students do not yet understand polarity, so this could be tricky)
Solution mixtures can only be liquids or gases.
Solution
Solutions can be a mixture between any of the following:
- liquids — liquids
- gases — gases
- gases — liquids
- solids — solids
- aqueous — liquids
- liquids — liquids
- gases — gases
- gases — liquids
- solids — solids
- aqueous — liquids
Which of the following mixtures is an alloy?
Solution
Video
An alloy is a solid solution mixture of 2 metals. The metals are mixed by melting the metals into the liquid state and mixing as a solution. Once cooled to a solid they are in the form you may be familiar with, like brass or stainless steel. Titanium and Gold are the only two mixtures of metals listed in the answer choices.
Pure Substances
Explain how pure water, made up of hydrogen (H) and oxygen (O) is considered a pure substance. [1]
Solution
Video
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Pure substances contain only 1 type of particle.
The H2O molecule is considered as one particle, and any number of these same particles is considered pure.
(If the hydrogen and oxygen were separated, then it would not be considered a pure substance because there would be different H2 and O2 particles).

Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
(If the hydrogen and oxygen were separated, then it would not be considered a pure substance because there would be different H2 and O2 particles).

Which of the following is a 'pure substance'?
Solution
Video
Only ice is made up of just 1 type of molecule/particle.
The rest of these things contain 2 or more different particles. Coins are now made of a combination of different metal elements. Paper contains cells that contain millions of different kinds of particles. Wood is made of cells that contain millions of different kinds of particles. Soap contains many different kinds of particles.
- Coin: alloy solution mixture of 2 or more metal elements
- Paper: contains cells with many elements (proteins, organelles, DNA, RNA, ions), also chemicals in processing
- Wood: contains cells with many elements (proteins, organelles, DNA, RNA, ions)
- Soap: many different ingredients, glycerol, oils, fragrances...
- Ice: is PURE WATER H2O and is the pure substance.
The rest of these things contain 2 or more different particles. Coins are now made of a combination of different metal elements. Paper contains cells that contain millions of different kinds of particles. Wood is made of cells that contain millions of different kinds of particles. Soap contains many different kinds of particles.
- Coin: alloy solution mixture of 2 or more metal elements
- Paper: contains cells with many elements (proteins, organelles, DNA, RNA, ions), also chemicals in processing
- Wood: contains cells with many elements (proteins, organelles, DNA, RNA, ions)
- Soap: many different ingredients, glycerol, oils, fragrances...
- Ice: is PURE WATER H2O and is the pure substance.
Properties & Characteristics
Physical State and Volume
Which of the following substances would contain particles the furthest distance apart, at a given temperature?
Solution
Video
Gases particles are farthest apart because the heat and kinetic energy overcomes the forces of attraction between the particles.


Quantitative and Qualitative Properties
What is the main difference between a qualitative and quantitative property? [1]
Solution
Video
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Qualitative properties are observations and do not include a numerical measurement like a quantitative property.
Qualitative properties are observed with the senses:
- Color, lustre, clarity
- Smell
- Texture, malleable, ductile, brittle
- Size
- Conductive to electricity
Quantitative properties are measured and are numeric:
- Mass
- Volume
- pH
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Qualitative properties are observed with the senses:
- Color, lustre, clarity
- Smell
- Texture, malleable, ductile, brittle
- Size
- Conductive to electricity
- Mass
- Volume
- pH
Which of the following is not a qualitative property?
Solution
Video
Qualitative properties are observations and do not include a numerical measurement like a quantitative property. Mass is an example of a quantitative property because it is usually measured.
Which of the following is a quantitative observation?
Solution
Quantitative observation are observations made by our senses like: sight, smell, etc.
Characteristics of Common Elements and Compounds
Which element has the following characteristic, at room temperature?
Solution
White and odourless
You may know that gold is yellow, and that chlorine has a smell.
Sodium is actually a shiny, lustrous metal in its pure form.
Neon gas is always orange (the different colors in neon signs are actually different gases than neon).
Calcium carbonate CaCO3 is a brittle/chalky, white substance with no odor. Many ionic salts form an odourless, white solid or powder.
Sodium is actually a shiny, lustrous metal in its pure form.
Neon gas is always orange (the different colors in neon signs are actually different gases than neon).
Calcium carbonate CaCO3 is a brittle/chalky, white substance with no odor. Many ionic salts form an odourless, white solid or powder.
Which element has the following characteristic at room temperature?
Solution
Shiny surface
Metals - including the alkali and alkaline earth metals - have a shiny luster, are malleable, ductile, and tend to conduct electricity and heat.
This may come as a surprise to you that pure sodium (Na) is actually a metal that people eat in food in small quantities called salts.
You should generally know
- Bromine is a halogen in group 17... it is not shiny
- Boron is a metalloid in group 13... it is not that shiny
- Hydrogen is a gas in group 1... it is not shiny
- Sodium is a metal in group 1... IT IS SHINY
- Carbon is a non-metal in group 14.. it is not shiny
This may come as a surprise to you that pure sodium (Na) is actually a metal that people eat in food in small quantities called salts.
You should generally know
- Bromine is a halogen in group 17... it is not shiny
- Boron is a metalloid in group 13... it is not that shiny
- Hydrogen is a gas in group 1... it is not shiny
- Sodium is a metal in group 1... IT IS SHINY
- Carbon is a non-metal in group 14.. it is not shiny
Differentiate Physical and Chemical Changes or Properties
You should learn the difference between chemical changes and physical changes (or chemical properties and physical properties).
A chemical change has occurred when bonds are broken, and new bonds and substances are formed.
Solution
A chemical change is when the atoms/bonds rearrange in some way.
The observational evidence that a chemical change has occurred includes: release (or absorption) of heat or light, odour, release of gas or bubbles, a change in colour, or formation of a new solid or liquid.
The observational evidence that a chemical change has occurred includes: release (or absorption) of heat or light, odour, release of gas or bubbles, a change in colour, or formation of a new solid or liquid.
Which of the following is a chemical change?
Solution
Remember that rusting is a chemical reaction/change because new bonds are formed as the molecules are rearranged.
Here are some examples of chemical changes:
- Combustion:
- Dissolving:
- Rust:
Here are some examples of chemical changes:
- Combustion:
- Dissolving:
- Rust:
Burning is an example of a chemical change.
Solution
Burning (combustion) changes the arrangement of the atoms to break or form new bonds. For example with methane gas,
Check the two examples of physical changes/properties.
Solution
Melting and boiling are physical changes because only a phase change occurs, without rearranging any bonds between atoms.
Dissolving a sugar is a physical change, not a chemical change because no bonds are breaking when the sugar dissolves in water. The sugar changes from a solid to aqueous phase.
Dissolving a salt is a chemical change because bonds are rearranged when the ionic compound breaks apart from a solid into aqueous ions in the water.
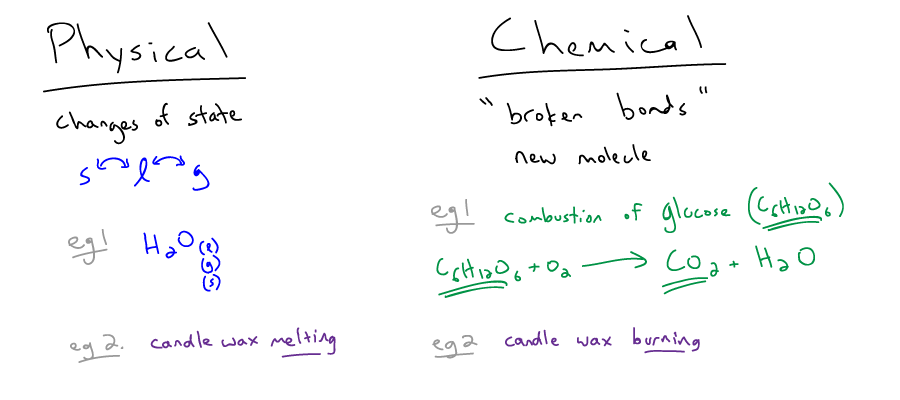
Dissolving a sugar is a physical change, not a chemical change because no bonds are breaking when the sugar dissolves in water. The sugar changes from a solid to aqueous phase.
Dissolving a salt is a chemical change because bonds are rearranged when the ionic compound breaks apart from a solid into aqueous ions in the water.

Explain why dissolving sodium chloride into water is an example of a chemical change, while dissolving glucose (sugar) into water is a physical change. [2]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Sodium chloride NaCl(s) is a salt, and the ionic bonds between the 'Na' cation and 'Cl' anion are broken apart when they dissociate in the solution. Chemical changes change the bonding of the molecules, while physical changes do not change the bonding.
See the difference between the two reaction equations... Only one breaks apart.
The sugar molecule C6H12O6 (s) has covalent bonds so does not break apart when it is dissolved (solvated) into water. Physical changes only change the phase state of a compound without rearranging/changing any bonding, e.g. just solid to aqueous, or gas to a liquid.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
The sugar molecule C6H12O6 (s) has covalent bonds so does not break apart when it is dissolved (solvated) into water. Physical changes only change the phase state of a compound without rearranging/changing any bonding, e.g. just solid to aqueous, or gas to a liquid.
Which two of the following cause a chemical change when dissolved in water?
Solution
Chemical changes are a change in the chemical bonding in a substance.
When ionic compounds dissolve in water, they separate into their aqueous (aq) ions (positive cation, and negative anion) and this is considered a chemical change.
H+Cl- and Na+Cl- are both ionic compounds.
When ionic compounds dissolve in water, they separate into their aqueous (aq) ions (positive cation, and negative anion) and this is considered a chemical change.
H+Cl- and Na+Cl- are both ionic compounds.
A student is mixing some chemicals in a safe, controlled laboratory. Two liquids are mixed without heating or cooling. Which of the following is not evidence that a chemical reaction has occurred, or is occurring?
Solution
Evaporation is a physical phase change from a liquid to a gas, therefore it is not a chemical reaction...
Other evidence a chemical reaction has occurred: change in temperature, production of light, or even odor...
Other evidence a chemical reaction has occurred: change in temperature, production of light, or even odor...
Sort the following chemical and physical changes and properties.
Solution
Melting
Color
Rusts
Covalent compound dissolves in water
Ionic compound dissolves in water
Solid, liquid, or gas phase
Combustible
Taste
Malleability or ductility
Burns
Density, mass, volume
Powder, or crystals
Chemical Change/Property
Physical Change/Property
Chemical Properties (based on reactions)
- Rusts
- Ionic compound dissolves in water
- Combustible
- Burns
- Density, mass, volume
- ...
Physical Properties (based on the senses)
- Melting
- Color
- Covalent compound dissolves in water
- Solid, liquid, or gas phase
- Taste
- Malleability or ductility
- Powder, or crystals
- ...
Melting
Color
Rusts
Covalent compound dissolves in water
Ionic compound dissolves in water
Solid, liquid, or gas phase
Combustible
Taste
Malleability or ductility
Burns
Density, mass, volume
Powder, or crystals
Chemical Change/Property
Physical Change/Property
- Rusts
- Ionic compound dissolves in water
- Combustible
- Burns
- Density, mass, volume
- ...
- Melting
- Color
- Covalent compound dissolves in water
- Solid, liquid, or gas phase
- Taste
- Malleability or ductility
- Powder, or crystals
- ...
Physical States
Given the melting and boiling points of certain substances below, determine which will be a solid at room temperature (23˚C). Solution Video
| Substance | Melting Point (˚C) | Boiling Point (˚C) |
| Water | 0 | 100 |
| Ammonia | -77 | -35 |
| Mercury | -38 | 357 |
| Olive Oil | −6 | 300 |
| Tar | 65 | 300 |
The solid will form when the room temperature (23˚C) is lower than the melting point. This is tar, which has a melting point at 65˚C.
Physical States of Matter: Phase Change
The process of turning a gas to a liquid is called vaporization.
Solution
Video

The process of turning a gas to a liquid is called condensation.


Physical States of Matter: Fluids
Which of the following are considered fluids?
Solution
- Solid
- Liquid
- Gas
Fluids expand to fill their container and have no fixed shape... this can be either a liquid or a gas... Many people don't realize gases are fluids (maybe because they confuse with the word liquid).
- Solid
- Liquid
- Gas
Density Intro
Density is calculated as:
A material floats when it has a lower density than the fluid.
Solution
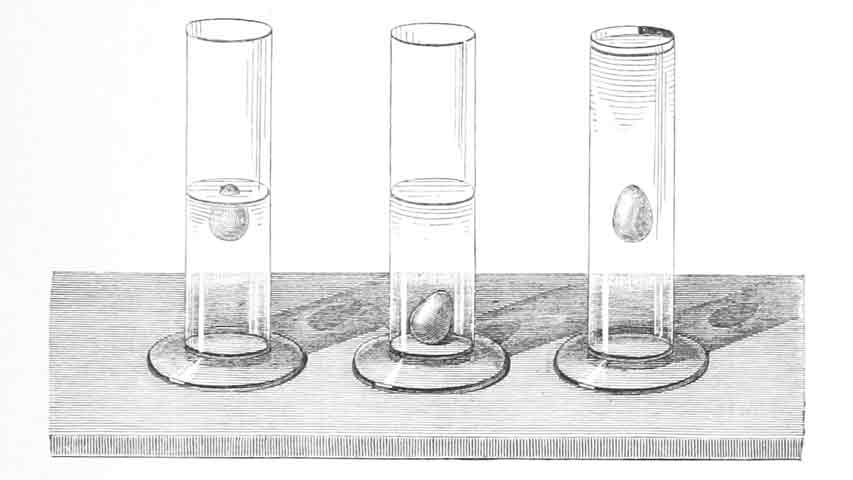
Materials with higher densities than the fluid density, will sink to the bottom because of gravity.
A 450 g object is dropped into a fluid that has a density of 6.5 g/cm3. If the object has dimensions 5 cm × 4 cm × 3 cm, then determine what will happen. (Show your work in your own notes.)
Solution
First calculate the volume:
Then calculate density:
Since the density of the object is higher than the fluid, then the object will sink.
When does something sink and when does it float? Solution
 |
(Don't accept mass or volume).
Objects sink when its density is greater than the density of the fluid... And visa versa...
Shown in the diagram from top to bottom...
Objects sink when its density is greater than the density of the fluid... And visa versa...
Shown in the diagram from top to bottom...
- Yellow: Oil
- Red: Wine alcohol
- Blue: Water with dye
- Green: Dish soap
- Brown: Maple syrup
The Unusual Density of Water
Water is most dense when it is:
Solution
Water is one of the few substances in the universe that is more dense as a liquid than as a solid.
Density Calculations
A 100 kg dinosaur bone is found in the desert.
Calculate the density, given the volume is 20 m3
Solution
Using your calculation of density, much would 4.25 m3 of this bone weigh?
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
kg
Hint Unavailable
Density,
Use density to calculate mass, given the volume,
Hint
Clear
Info
Incorrect Attempts:
CHECK
kg
Hint Unavailable
Atoms, Elements, & Periodic Table
Introduction to The Elements
Familiarize yourself with the periodic table.
Which of the following elements is a non-metal?
Solution
Nitrogen is a non-metal
Which of the following contains all metals?
Solution
All metals: Calcium, Magnesium, Lithium, Sodium
Which of the following is not an element?
Solution
Elements are the most basic unit of a substance and cannot be divided into other substances. Steel is an alloy made of more than one element.
What do the elements have in common?
Solution
Ca, Cl, C
- Ca is Calcium, a metal
- Cl is Chlorine, gas and non-metal
- C is Carbon, a non-metal
- Ca is Calcium, a metal
- Cl is Chlorine, gas and non-metal
- C is Carbon, a non-metal
The Periodic Table
The periodic table is organized according to:
Solution
Atomic number, the number of protons!
A row on the periodic table is called a group.
Solution
A row on the periodic table is called a period. A column is a group.
Elements in the same group tend to share similar physical and chemical properties.
Solution
Elements that share a group are in the same chemical family and share physical properties because they have the same number of valence electrons. The chemical property is based on the number of valence electrons that elements in a certain group have.
Which of the following elements would have similar physical and chemical properties to Neon (Ne)?
Solution
Because elements in the same group tend to share similar physical and chemical properties. Both Neon and Argon are in group 18.
Which collection of atomic numbers of elements is likely to share the same physical and chemical properties?
Solution
Elements in the same group tend to share similar physical and chemical properties. Elements with atomic numbers 1, 3, 11, 19 are in the same group, group 1.
All these elements in the same group 1 would react to lose 1 electron to form +1 charged cations. For example:
H+1, Li+1, Na+1, K+1
All these elements in the same group 1 would react to lose 1 electron to form +1 charged cations. For example: H+1, Li+1, Na+1, K+1
The Chemical (Group) Family on the Periodic Table
You can use a periodic table for the following...
Alkali earth metals are located in group:
Solution
Video
The main names are: alkali earth metals (group 1: Li, Na, K...), alkaline earth metals (group 2: Be, Mg, Ca...), ..., halogens (group 17: F, Cl, Br...), and noble gases (group 18: He, Ne, Ar...).
Alkali earth metals are located in group 1.
Alkali earth metals are located in group 1.
Determine the different group numbers that each of the following chemical families are in: Alkali earth metals, Alkaline earth metals, Halogens, and Noble gases.
Solution
Alkali earth metals: Group 1
Alkaline earth metals: Group 2
Halogens: Group 17 (or 7A)
Noble gases: Group 18 (or 8A)
Alkaline earth metals: Group 2
Halogens: Group 17 (or 7A)
Noble gases: Group 18 (or 8A)
The following elements belong to which chemical family (group)? Solution
 |
Alkaline earth metals
The elements of which chemical family (group) are most reactive in water?
Solution
Alkali earth metals are most reactive in water. This is called a physical property.
The elements of which chemical family (group) are inert, and tend not to react chemically?
Solution
Noble gases are considered inert, mostly.
Chlorine (Cl) is a gas at room temperature and has a green-yellow colour. Which chemical family (group) does chlorine belong to?
Solution
Halogens
Classify the following 7 elements...
Solution
B, Si, Ge, As, Sb, Te, Po
These are the 7 Metalloids. Metalloids are special elements that have a mixture of properties of metals and non-metals. They are located on a step-ladder (staircase) on the periodic table between the metals and the non-metals.
Metals have a shiny luster, are malleable, ductile, and tend to conduct electricity and heat... But metalloids only have some combinations of these properties. For example Silicon (Si) has a shiny luster, but is not malleable, is brittle, and is a poor conductor of electricity...
Metals have a shiny luster, are malleable, ductile, and tend to conduct electricity and heat... But metalloids only have some combinations of these properties. For example Silicon (Si) has a shiny luster, but is not malleable, is brittle, and is a poor conductor of electricity...
Components of the Atomic Model
Electrons located on the outermost energy level of an atom are called the:
Solution
Neutrons are...
Solution
Neutrons are neutral particles found in the nucleus (in the center) of an atom.
Atomic Structure and the Periodic Table
Copy the table below and fill in the spaces with the atomic structures showing the following things.
Solution
- Number of protons and neutrons in the nucleus
- The electrons on each ring (energy level), including the valence


- Number of protons and neutrons in the nucleus
- The electrons on each ring (energy level), including the valence

On the periodic table, going across a period (left-to-right) increases the number of ________(I)________, and going down a group (top-to-bottom) increases the number of ________(II)________. [Not referring to protons or neutrons]
Solution
Hint
Clear
Info
I =
II =
Incorrect Attempts:
CHECK
Hint Unavailable
On the periodic table, going across a period (left-to-right) increases the number of valence electrons on the outer orbit, and going down a group (top-to-bottom) increases the number of energy levels or orbits/rings.
Hint
Clear
Info
I =
II =
II =
Incorrect Attempts:
CHECK
Hint Unavailable
Atomic Structure and the Periodic Table
Decipher the hidden messages below, given the atomic structures.
Solution
 Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Using atomic structure diagrams, you should get:
OCCaSiONAl CaBS HONK ON PHONe LiNeS
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Using atomic structure diagrams, you should get:
OCCaSiONAl CaBS HONK ON PHONe LiNeS
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Solution
 Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Using atomic structure diagrams, you should get:
BeN POPS OFF SOCKS SO He CaN COOK NaCHOS
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Using atomic structure diagrams, you should get:
BeN POPS OFF SOCKS SO He CaN COOK NaCHOS
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Atomic Number and Atomic Mass
The number of neutrons always equals the number of protons in an atom.
Solution
The number of neutrons is calculated with:
Atomic Mass - Atomic Number
The atomic number always equals the number of protons in an atom.
Solution
True
It is possible for the atomic mass to be smaller than the atomic number.
Solution
The atomic mass is always higher than the atomic number. The atomic mass equals the number of protons + neutrons. The atomic number equals only the number of protons.
A Carbon atom has the following quantities: atomic number = 6, atomic mass = 12. Determine the number of neutrons in one Carbon atom.
Solution
Video
A Silicon atom has 14 neutrons, 14 electrons, and 14 protons. What is the atomic mass of Silicon?
Solution
Video
Atomic mass = Number of Protons + Number of Neutrons
Atomic mass = 14 + 14
Atomic mass = 28
Atomic mass = 14 + 14
Atomic mass = 28
Given the neutral (uncharged) element from the periodic table for Potassium (K), determine the number of protons, electrons, and neutrons, respectively.
Solution
19 = atomic number = number of protons
39 = atomic mass = number of protons + neutrons
A neutral (uncharged) element has:
number of protons = number of electrons = 19
39 = atomic mass = number of protons + neutrons
A neutral (uncharged) element has: number of protons = number of electrons = 19
Which element would have a half-full second energy level?
Solution
The second energy level can hold up to 8 electrons. Carbon has 4 electrons in the second energy level, the electrons on this outer shell are also known as valence electrons.
An isotope of an atom has:
Solution
Video
The atomic number (number of protons) of an atom can never change - otherwise we would be able to convert one element into another, like convert iron into gold!
These two elements are the exact same in every way, except for one thing...
 Isotopes have a different number of neutrons. (But a different number of electrons is called an ion, rather than an isotope.)
Isotopes have a different number of neutrons. (But a different number of electrons is called an ion, rather than an isotope.)
These two elements are the exact same in every way, except for one thing...
 Isotopes have a different number of neutrons. (But a different number of electrons is called an ion, rather than an isotope.)
Isotopes have a different number of neutrons. (But a different number of electrons is called an ion, rather than an isotope.)
The Thompson Model of an Atom
The Thompson model of an atom consists of a positively charged sphere with negatively charged electrons distributed evenly throughout the sphere.
Solution
Yes this was how Thompson thought the atom was made. Thompson's model spaced the negatively charged particles evenly throughout a positively charged substance in the atom, like a "plum pudding", or chocolate chips in a cookie. (But he was eventually proven wrong about this atomic model)...
Geiger–Marsden (Rutherford's) Gold Foil Experiment
Rutherford's gold foil experiment demonstrated that
Solution
Atoms contain a cluster of positively charged protons located at the center. (He was right, and Thompson was wrong)...
Rutherford shot particles (in the form of alpha particles with a mass) at gold foil, what he noticed was the particles were not all traveling through the gold atoms evenly.
Sometimes the particles were being reflected backwards. Therefore there must have been something inside the atom with a significant mass, and this they later found was the nucleus (so they discovered that atoms have a nucleus). The electrons don't have enough mass to reflect the alpha particles.
Rutherford shot particles (in the form of alpha particles with a mass) at gold foil, what he noticed was the particles were not all traveling through the gold atoms evenly.
Sometimes the particles were being reflected backwards. Therefore there must have been something inside the atom with a significant mass, and this they later found was the nucleus (so they discovered that atoms have a nucleus). The electrons don't have enough mass to reflect the alpha particles.
Bohr-Rutherford Model of an Atom
In an atom, electrons are located:
Solution
Draw a Bohr-Rutherford model of a Calcium (Ca) atom. (In your own notes)
Solution
[Not shown.] Your diagram should include the central charges (20 p+, and 20n) and four rings of electrons with 2 unpaired on the inner, 8 (4 pairs) on the second, 8 (4 pairs) on the third, and 2 unpaired electrons on the outer/valence orbit.
Applications of The Bohr-Rutherford Model of an Atom
The innermost orbit of an atom can contain up to 8 electrons.
Solution
Video
The innermost orbit on an atom can contain up to 2 electrons.
Bohr-Rutherford models of the elements in the halogen group would have which of the following in common?
Solution
Number of electrons on the outer ring
Which element would have the same number of orbits (rings) as Sodium, Na?
Solution
Elements in the same period (row) have the same number of orbits.
An element with an atomic number of 12 has what numbers of electrons on each orbit, ordered from inside to outside?
Solution
The element with atomic number 12 is Magnesium (Mg). This is in the 3rd period (row), which means there are 3 energy levels...
= 2, 8, 2
= 2, 8, 2
Atoms
The electrical conductivity of metals depends on which part of an atom?
Solution
Electrical conductivity is based on the movement of electrons.
Ions
What is an ion? [1]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
An ion is a charged particle that forms from a neutral element when electrons are removed or added. Elements that lose electrons form positive cations. Elements that gain electrons form negative anions.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
If a Calcium (Ca) ion has a charge of 2+, determine the total number of electrons, given the atomic number of Calcium is 20.
Solution
18 electrons
Which of the following is an ion?
Solution
An ion has a charge, either as a positive cation, or a negative anion.
Which of the following elements will form an anion?
Solution
Non-metals that gain electrons form negatively charged anions. These elements are located on the upper-left side of the periodic table.
O2-
(Hydrogen, potassium, and aluminum form cations. Argon does not form an ion because noble gases are inert).
Determine the number of electrons that Magnesium will lose when a Magnesium cation is formed, and that Sulfur will gain when a Sulfur anion is formed.
Solution
Hint
Clear
Info
Magnesium loses:
Sulfur gains:
Incorrect Attempts:
CHECK
electrons
Hint Unavailable
When Magnesium loses electrons to form its cation form... It will lose all the electrons in the outer valence orbit (energy level). Magnesium has 2 electrons in the valence that it will lose because it is in group 2.
When Sulfur gains electrons to form its anion form... It will gain all the electrons it needs to fill the outer valence orbit (energy level) up to 8 electrons. Sulfur starts with 6 electrons in the valence because it is in group 6A (or 16), so it needs to gain 2 more electrons.
Hint
Clear
Info
Magnesium loses:
Sulfur gains:
Sulfur gains:
Incorrect Attempts:
CHECK
electrons
Hint Unavailable
When Sulfur gains electrons to form its anion form... It will gain all the electrons it needs to fill the outer valence orbit (energy level) up to 8 electrons. Sulfur starts with 6 electrons in the valence because it is in group 6A (or 16), so it needs to gain 2 more electrons.
Explain why Chlorine has a -1 charge when it forms an ion. [1]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
The -1 charge comes from the difference in protons and electrons in the chloride ion.
Chlorine starts with 17 protons and 17 electrons. When the chloride ion gains 1 electron, it then has 17 positively charged protons and 18 negatively charged electrons. One more electron makes the charge -1.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Chlorine starts with 17 protons and 17 electrons. When the chloride ion gains 1 electron, it then has 17 positively charged protons and 18 negatively charged electrons. One more electron makes the charge -1.
Neutral, uncharged Lithium (Li) has an atomic number of 3 and a mass number of 7. For the Lithium ion, determine the number of protons, electrons, and neutrons, respectively.
Solution
3 = atomic number = number of protons
7 = atomic mass = number of protons + neutrons
A neutral (uncharged) element has: number of protons = number of electrons = 3
But the Lithium ion is in group 1 so it will lose 1 electron to get a +1 charge.
A positively charged element has:
number of electrons = number of electrons - number electrons lost
= 3 - 1
= 2 electrons.
7 = atomic mass = number of protons + neutrons A neutral (uncharged) element has: number of protons = number of electrons = 3
But the Lithium ion is in group 1 so it will lose 1 electron to get a +1 charge. A positively charged element has:
number of electrons = number of electrons - number electrons lost
= 3 - 1
= 2 electrons.
Combining Atomic Mass, Atomic Number, and Ions
Given some of the details in the tables, determine the missing values.
Solution
Hint
Clear
Info
Element Charge Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons
4 5 2
Incorrect Attempts:
CHECK
Hint Unavailable
- Number of protons is the atomic number... 4.
- Number of protons tells us the element is Beryllium.
- +4 protons and -2 electrons makes a +2 charge.
- Mass number is protons + neutron so... 9.
Element Charge Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons
Beryllium +2 4 9 4 5 2
Hint
Clear
Info
| Element | Charge | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
| 4 | 5 | 2 |
Incorrect Attempts:
CHECK
Hint Unavailable
- Number of protons is the atomic number... 4.
- Number of protons tells us the element is Beryllium.
- +4 protons and -2 electrons makes a +2 charge.
- Mass number is protons + neutron so... 9.
| Element | Charge | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
| Beryllium | +2 | 4 | 9 | 4 | 5 | 2 |
Solution
Hint
Clear
Info
Element Charge Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons
-2 16 16
Incorrect Attempts:
CHECK
Hint Unavailable
- Number of protons is the atomic number... 16.
- Number of protons tells us the element is Sulfur.
- Charge tells us there are 2 more electrons then protons so... 18 electrons.
- Mass number is protons + neutron so... 32.
Element Charge Atomic Number Mass Number Number of Protons Number of Neutrons Number of Electrons
Sulfur -2 16 32 16 16 18
Hint
Clear
Info
| Element | Charge | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
| -2 | 16 | 16 |
Incorrect Attempts:
CHECK
Hint Unavailable
- Number of protons is the atomic number... 16.
- Number of protons tells us the element is Sulfur.
- Charge tells us there are 2 more electrons then protons so... 18 electrons.
- Mass number is protons + neutron so... 32.
| Element | Charge | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
| Sulfur | -2 | 16 | 32 | 16 | 16 | 18 |
COMPOUNDS
Characteristics of Common Elements and Compounds
CHCl3 is an atom.
Solution
CHCl3 is a molecule or compound consisting of five atoms: one carbon, one hydrogen, and three chlorine atoms.
Which of the following is not a chemical compound?
Solution
A compound is composed of two or more elements. A compound is not found on the periodic table. Potassium is the only single element listed, i.e. not a compound.
The Seven Diatomic Molecules
Which of the following does not form a diatomic molecule?
Solution
(Note that diatomic molecules are not considered compounds.) The seven diatomic molecules you need to know are, "HOFBRINCL": H2, O2, F2, Br2, I2, N2, Cl2.
Covalent Compounds
Two non-metals combine to form a covalent compound.
Solution
True
Glucose is a simple sugar with the chemical formula, C6H12O6. How many non-metal atoms are in one molecule of glucose?
Solution
6 Carbons + 12 Hydrogens + 6 Oxygens = 24 atoms
Predict which of the following elements will form a covalent compound when combined.
Solution
Covalent compounds are formed when two non-metals combine and share electrons.
(An exception is like hydrogen ion plus chlorine ion).
(An exception is like hydrogen ion plus chlorine ion).
Ionic Compounds
An ionic compound consists of the combination of one neutral atom, plus one charged atom (anion or cation).
Solution
Ionic compounds consist of a positively charged ion (cation), combined with a negatively charged ion (anion). The cation loses one or more electrons to the anion.
Determine the correct statement regarding the ionic compound, MgCl2.
Solution
Magnesium is a metal.
Chlorine is a non-metal.
The compound contains three atoms and two different elements.
Chlorine is a non-metal.
The compound contains three atoms and two different elements.
Which of the following is an ionic compound?
Solution
An ionic compound is always made up of charged atoms, or ions. Usually an ionic compound consists of a metal cation, and a non-metal anion.
An ionic compound called aluminum trioxide is formed when two aluminum atoms combine with three oxygen atoms. Show the chemical formula for one molecule of aluminum trioxide. Determine which atom is the cation. [2]
Solution
⁰
¹
²
³
⁴
⁵
⁶
⁷
⁸
⁹
⁻
⁺
⁽
⁾
₀
₁
₂
₃
₄
₅
₆
₇
₈
₉
₋
₊
₍
₎
Incorrect Attempts:
CHECK
Hint Unavailable
Al2O3
The positively charged Aluminum metal ion is the cation, and the negatively charged Oxygen non-metal ion is the anion.
The cation is always written first, and the anion last, in the chemical formula of an ionic compound.
⁰
¹
²
³
⁴
⁵
⁶
⁷
⁸
⁹
⁻
⁺
⁽
⁾
₀
₁
₂
₃
₄
₅
₆
₇
₈
₉
₋
₊
₍
₎
Incorrect Attempts:
CHECK
Hint Unavailable
The positively charged Aluminum metal ion is the cation, and the negatively charged Oxygen non-metal ion is the anion.
The cation is always written first, and the anion last, in the chemical formula of an ionic compound.
Identifying Ionic and Covalent Compounds
This year, you only need to be able to identify compounds from their formulas - you don't need to know how to name them.
Distinguish between the ionic and covalent compounds by dragging the formulas into their category.
Solution
Water
H2O
Lithium bromide
LiBr
Oxygen
O2
Ammonia
NH3
Sodium chloride
NaCl
Methane
CH4
Carbon dioxide
CO2
Magnesium oxide
MgO
Ionic
Covalent
Remember ionic is: metal + non metal
Covalent: non metal + non metal.
Water
H2O
H2O
Lithium bromide
LiBr
LiBr
Oxygen
O2
O2
Ammonia
NH3
NH3
Sodium chloride
NaCl
NaCl
Methane
CH4
CH4
Carbon dioxide
CO2
CO2
Magnesium oxide
MgO
MgO
Ionic
Covalent
Covalent: non metal + non metal.
Order the ionic and covalent compounds from heaviest (at the top) to lightest (at the bottom). Remember to use atomic mass.
Solution
NH3
CH4
CO2
MgO
NaCl
H2O
LiBr
O2
Look up the atomic masses on the periodic table (the bigger number) is g/mol...
- LiBr = 86.84 g/mol
- NaCl = 58.44 g/mol
- CO2 = 44.01 g/mol
- MgO = 40.31 g/mol
- O2 = 32.00 g/mol
- H2O = 18.02 g/mol
- NH3 = 17.04 g/mol
- CH4 = 16.05 g/mol
NH3
CH4
CO2
MgO
NaCl
H2O
LiBr
O2
- LiBr = 86.84 g/mol
- NaCl = 58.44 g/mol
- CO2 = 44.01 g/mol
- MgO = 40.31 g/mol
- O2 = 32.00 g/mol
- H2O = 18.02 g/mol
- NH3 = 17.04 g/mol
- CH4 = 16.05 g/mol
Distinguishing Ionic and Covalent Compounds
A white chalky powder is left over in a lab from a chemical reaction. A curious chemist wants to quickly see if the compound is ionic or covalent. Determine based on the evidence below, whether the compound is ionic or covalent.
Solution
- The chemist heats the compound over very high heat at 300˚C and the compound does not burn or melt.
- The chemist then mixes the compound with water and it dissolves easily.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Ionic compounds have a high melting point and are mostly soluble in water. Covalent compounds have a low melting point, and are often insoluble in water. Covalent compounds will burn at lower temperatures, since the compound did not melt or burn, then it is most likely ionic.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Earth & Space Science: the Universe
Moon
The Earth is the only planet in our solar system that has a moon.
Solution
The Earth only has one moon.
Jupiter has 63 moons!
Other planets have moons too, Saturn has 62 moons, Mars has 2 moons, ... and more...
Jupiter has 63 moons!
Other planets have moons too, Saturn has 62 moons, Mars has 2 moons, ... and more...
Moon
The same side of Earth's Moon always faces Earth because...
Solution
The Moon does rotate on its axis, and it does orbit the Earth.
The time it takes the Moon to orbit the Earth (the orbital period) is the same amount of time it takes for the Moon to rotate on its axis.
Therefore we always see the same side of the moon, even though the Moon is rotating on its axis. (BTW, the Moon's orbital period, and rotation period is 27.3 days).
The time it takes the Moon to orbit the Earth (the orbital period) is the same amount of time it takes for the Moon to rotate on its axis.
Therefore we always see the same side of the moon, even though the Moon is rotating on its axis. (BTW, the Moon's orbital period, and rotation period is 27.3 days).
Inner Earth Core
The center of the Earth (core) is almost as hot as the surface of the Sun.
Solution
(This fact is slightly less known by students.) The core of the Earth is molten metal and rock because it is so hot. The temperature of the core of the Earth is close to the temperature of the surface of the Sun, around 5,430 °C. (Of course the core of the sun is many, many times hotter!)
Moon Terminology
The moon is classified as a type of...
Solution
A satellite travels around a planet.
Solar System Order
The Milky Way is our solar system.
Solution
The Milky Way is our galaxy, which contains our solar system (the Sun + 8 planets).
Dwarf Planets
Dwarf planets orbit a central star and are generally spherical in shape.
Which of the following is considered a dwarf planet?
Solution
Pluto
Why is it (above) considered a dwarf planet (what is a dwarf planet)? [1]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Pluto is a dwarf planet because its orbit crosses paths with another larger planet, Neptune.
Since Pluto is not the largest mass in this shared orbit, it is only considered a dwarf planet.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Since Pluto is not the largest mass in this shared orbit, it is only considered a dwarf planet.
Asteroids and Meteoroids
What is the main difference between an asteroid and a meteoroid?
Solution
Meteoroids are smaller than asteroids. Asteroids are like tiny planets, which also orbit the Sun like meteoroids. Meteoroids are debris formed from the collision of larger asteroids, or planets, etc. (Both terms are used for the presence in space, and are made of rock and metal)
Meteoroids
As a meteoroid falls from space to the Earth, which is the correct order of the name as it falls from space, to the ground? Solution
 |
meteoroid (in space)
↓
meteor (in sky/atmosphere)
↓
meteorite (on ground)
(A 'shooting star' in the sky is a meteor).
↓
meteor (in sky/atmosphere)
↓
meteorite (on ground)
(A 'shooting star' in the sky is a meteor).
Comets
What is a comet? [2]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Comets are clumps of ice, dust, and rock that orbit the Sun.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Bodies
Meteoroids, asteroids, and comets all orbit the Sun.
Solution
True.
Celestial Bodies
Determine what is wrong with the following statement. [1]
Solution
The terrestrial planets are the 4 inner planets, and the gaseous planets are the 5 outer planets.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
The inner 4 planets are terrestrial (rock-based), while the outer 4 are gaseous based.
(There are only 8 planets that orbit the Sun).
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
(There are only 8 planets that orbit the Sun).
Celestial Bodies
State one similarity and one difference between gaseous planets and terrestrial planets. [2]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
(Answers may vary...)
Both have gravity and spherical in shape.
Gaseous planets made of gas, and terrestrial is sold rock/metal based.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Both have gravity and spherical in shape.
Gaseous planets made of gas, and terrestrial is sold rock/metal based.
Understanding Powers of Ten with Scientific Notation
Change the following scientific notation to 'regular' numbers.
1.634 × 10-9
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
2.50 × 104
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Understanding Powers of Ten with Scientific Notation
Convert to Scientific Notation.
0.0000519
Solution
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
50,525
Solution
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
Understanding Powers of Ten with Scientific Notation: Multiplication and Division
Evaluate.
(1.20 × 107) x (2.00 × 105)
Solution
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
(3.0 × 108) ÷ (1.5 × 1012)
Solution
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
Hint
Clear
Info
× 10
Incorrect Attempts:
CHECK
Hint Unavailable
Astronomical Units
You know that the distance from the Sun to the planets is very far. So, the term astronomical unit (AU) is used to simplify this very far distance.
| Planet | Astronomical Units (AU) |
| Venus | 0.39 |
| Earth | 1.00 |
| Mars | 1.52 |
| Neptune | 30.06 |
One astronomical unit is the
Solution
1 Astronomical Unit (AU)
= 149, 597, 871 kilometers
≈ 150, 000, 000 kilometers
≈ 150 × 106 kilometers
= 149, 597, 871 kilometers
≈ 150, 000, 000 kilometers
≈ 150 × 106 kilometers
Mars is 1.52 astronomical units (AU) and the Earth is 1.00 AU. This means that if the planets are aligned, the distance between these planets is 0.52 AU
Solution
True
Given the value of an astronomical unit below, determine the distance between Venus and Earth, in kilometers.
Solution
1 AU ≈ 150 × 106 km
Light Year
One light year (ly) is equal to approximately, 9.5 × 1012 km.
One ly is also equal to 9.5 × 1015 m
Solution
There are 1,000 m (103 m) in 1 km... So 9.5 × 1012 km => 9.5 × 1012 + 3 m => 9.5 × 1015 m
If the distance from Earth to the center of our Milky Way galaxy is 2.47 × 1015 km, calculate the distance in light years.
Solution
1 ly = 9.5 × 1012 km
Orbital Radius
The orbital radius is the
Solution
The radius of the orbit of the Earth around the Sun is the distance from the Sun to the Earth.
Orbit
What causes the orbit of planets around the Sun?
Solution
Gravity from the Sun prevents the planets in our solar system from going off in a straight path.
The Sun
The temperature of the surface of the Sun is ~6,000 ˚C while the core temperature is ~15 million ˚C.
Solution
The surface of the Sun is, relatively, much cooler than its core. Nuclear fusion occurs in the core of the Sun, making it really hot [equivalent in energy to detonating 400 billion megaton nuclear bombs, every second]. But the surface of the Sun is actually nearly the same temperature of the core of the Earth.
The Sun could fit (approximately) how many Earths inside of it?
Solution
Approximately 1.3 million Earths would fit inside the Sun.
Explain the cause of Northern Lights (Aurora Borealis). [2]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Charged particles (ions) escape from the surface of the Sun, traveling towards Earth. These particles are affected by the magnetic field and enter the atmosphere near the poles. The ions interact with atoms in the atmosphere, and this creates the photons of light that we see as the Northern Lights (Aurora Borealis).
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
The main source of energy in the Sun is...
Solution
The main source of energy is nuclear fusion, of Hydrogen gas into Helium gas, occurring in the core at 15 million Kelvin. 600 - 700 million tons of Hydrogen is converted into helium, each second!
The mass of the Sun, is mainly due to...
Solution
The mass of the Sun is largely due to Hydrogen. By mass, the Sun is 75 percent Hydrogen, and 25 percent Helium. Metals make up less than 0.1 percent of the mass of the Sun.
Sun and Moon, Rise and Set
The Sun rises in the east and sets in the west, while the Moon rises in the west and sets in the east.
Solution
Both the Sun and the Moon rise in the east and set in the west. (This is lesser known by students)
Models of the Universe
The current model of the universe is called the Geocentric model.
Solution
The current model of the universe is called the Heliocentric model, in which the sun is at the center of the solar system.
The Axis of the Earth, Application
If the Earth was not tilted as it revolves on a 23.5˚ axis, then which of the following features would no longer occur?
Solution
The difference seasons are due to the tilt of the Earth, causing the North and South hemispheres to receive differing amounts of light in summer and winter during Earth's orbit.
Causes of Seasons
 |
The following diagram (not to scale) depicts the Earth during part of its orbit around the Sun. Which of the following is true?
Solution

Summer in the north:
During daytime, the Sun's light rays hit the northern hemisphere of the Earth more directly than the south. More intense light causes higher temperatures––summer.
Winter in the south:
During daytime, the Sun's light rays are weaker in the southern hemisphere as this part is angled more away from the Sun.
During daytime, the Sun's light rays hit the northern hemisphere of the Earth more directly than the south. More intense light causes higher temperatures––summer.
Winter in the south:
During daytime, the Sun's light rays are weaker in the southern hemisphere as this part is angled more away from the Sun.
Sketch a diagram to show the orbit position, and tilt of the Earth in the fall and winter in the northern hemisphere. Include the Earth, Sun, and direction of tilt of Earth's axis in your diagram.
Solution



Lunar Cycle
Describe what is happening (depicted) in the following lunar cycle diagram, where the Moon is shown in different phases orbiting the Earth, in relation to the Sun.

Lunar Eclipse
A lunar eclipse occurs when...
Solution
During a lunar eclipse, the Earth blocks the light rays coming from the Sun - the Moon is in Earth's shadow.
(Also note that a solar eclipse occurs when the Moon is between the Earth and the Sun - the moon blocks the light coming from the Sun).
(Also note that a solar eclipse occurs when the Moon is between the Earth and the Sun - the moon blocks the light coming from the Sun).
Tides
Describe how tides are formed. [1] Solution
 |
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Gravity from the Moon pulls the water on Earth.
Gravity
Gravity is caused by celestial bodies with large volumes.
Solution
Gravity is caused by large masses. Objects with high volumes do not always have high masses (this is low density).
Gravity depends on the mass.
Gravity depends on the mass.
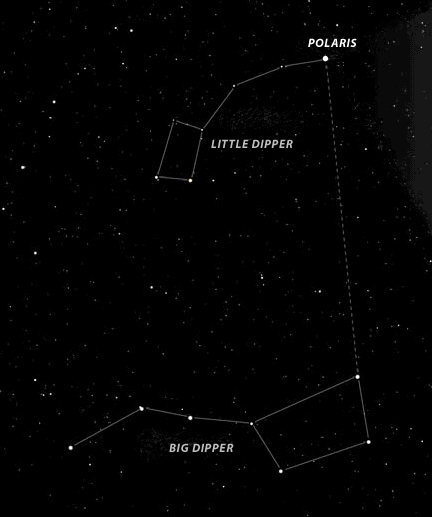
Constellations
Stars, as seen in the sky, are not moving in space.
Solution
Technically, the stars are moving because the universe is expanding. However, the movement cannot be noticed when you see the stars in the sky. The difference would be noticeable to the eye over 10,000 years. This is not to be confused with the 'apparent motion of the stars due to the movement (rotational, translational) of the earth.'
 |
North Star
Which is the North Star?
Solution
Polaris is the North Star. It is significant because it is located above the north pole in the northern hemisphere, and stays in one place.
NASA, ESA, N. Evans (Harvard-Smithsonian CFA), and H. Bond (STScl)
 |
Satellites
The Moon is a satellite.
Solution
A satellite is a body orbiting a planet. There are natural satellites such as the Moon, and artificial satellites, such as GPS and radio broadcasters.
Satellites Orbit
Which of the following factors affect the orbit of a satellite?
Solution
Mass and speed
Orbits
Which of the following orbits is fixed in place over the Earth?
Solution
Objects in a geosynchronous orbit stay in one spot in the sky, relative to a position on the Earth.
If you have a satellite television network, next time think that your signal is broadcast up 38,000km and back down 38,000 km for a total distance of about 76,000 km.
If you have a satellite television network, next time think that your signal is broadcast up 38,000km and back down 38,000 km for a total distance of about 76,000 km.
Retrograde Motion
What causes retrograde motion?
Solution
The speed of planets
Star Temperatures and Color
Order star color from hottest to coolest (top to bottom)
Solution
White
Blue
Red
Yellow
From hottest to coolest:
Blue, White, Yellow, Red
Blue is about 40,000˚C, Red is about 3,000˚C.
White
Blue
Red
Yellow
Star Energy
A star's luminescence is the glow of gases due to very high temperatures that heat the gases on the surface of the star. What creates the energy in a star that makes the high temperature?
Solution
Nuclear fusion is when hydrogen (H) atoms are pressed together under high pressure at the center of gravity of the star to form new atoms of Helium (He) and others. Very high amounts of energy are released from the center of stars as new atoms form. This travels outwards to create a glowing surface, with the difference colors based on the gases and the temperature.
Apparent and Absolute Magnitudes of Luminosity
Two different light sources, a very bright lamp, and a dim flashlight are held different distances from someone who can see the different brightness of the sources. The perceived brightness of a light source is luminosity.

It is possible for the dimmer flashlight to appear brighter than the lamp, depending on the distance to the observer.
Solution
True
Which statement is correct about apparent magnitude and absolute magnitude ?
Solution
Absolute magnitude is the strength of the light source, and apparent magnitude is how bright it appears to the observer
Explain how apparent magnitude and absolute magnitude describes the luminosity of stars at different distances.
Solution
- Apparent magnitude is
- Absolute magnitude is constant for a star and is related to its energy emitted.
It is possible for a star with a higher absolute magnitude to have a lower luminosity than a star with a low absolute magnitude. How? --> If the star with a low absolute magnitude is significantly closer to the observer...
- Apparent magnitude is
- Absolute magnitude is constant for a star and is related to its energy emitted.
Hertzsprung-Russell Diagram
Hertzsprung-Russell diagrams plot:
Solution
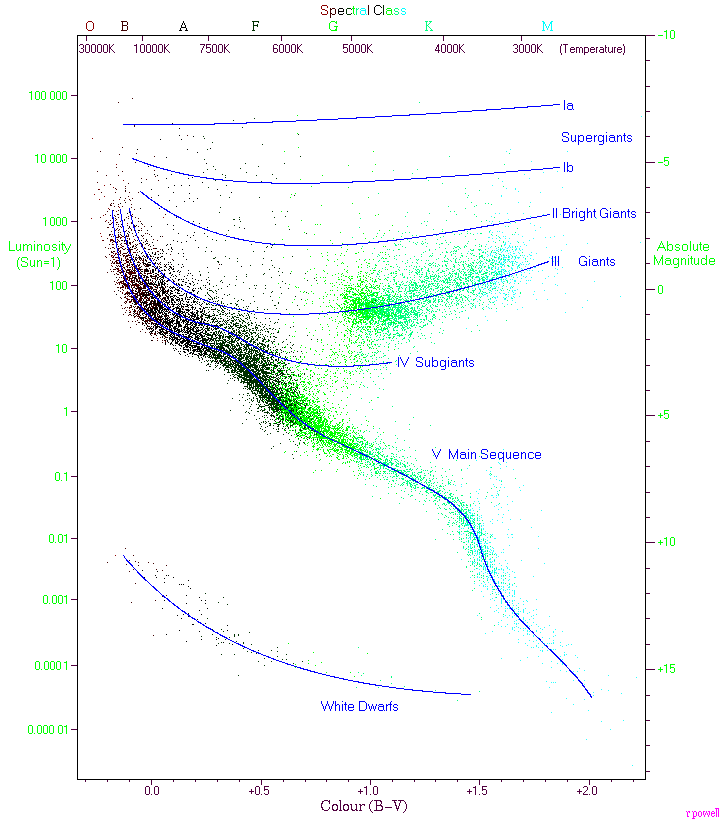
CC Credit: Hipparcos Catalogue from the Gliese Catalogue of Nearby Stars
 |
Hertzsprung-Russell Diagram Star Location
The Hertzsprung-Russell (HR) diagram tells us the color and absolute luminosity of stars.
Where are red giants located on an HR diagram?
Solution
Red giants have low temperature, and high absolute luminosity.
Where are white dwarfs located on an HR diagram?
Solution
White dwards have high temperature, and low absolute luminosity.
Our Sun is located outside of the main sequence on the HR diagram
Solution
Our Sun is located in the main sequence.
Life Cycle of Stars
What is the next phase of life of a red giant star?
Solution
Main sequence →→ Red Giant →→ White Dwarf →→ Nebula
Main sequence →→ Blue/Red Supergiant →→ Supernova →→ Nebula →→ Neutron Star
Main sequence →→ Blue/Red Supergiant →→ Supernova →→ Nebula →→ Neutron Star
Nebula
Nebulas form new stars
Solution
Nebula provides the gas matter to make stars. (Nebulas are also formed at the end of the life cycle of a star.) And they look pretty awesome:

NASA, via Antony McAulay
 |
Life Cycle of Stars
Describe how new elements are formed in a star. [2]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Fusion of Hydrogen and Helium. When the supply of Hydrogen (H) is used up, then the Helium (He) is used for fusion. Helium results in heavier atoms with more protons, like Carbon (C), Nitrogen (N), Oxygen (O), ...
(As an aside, the heavier elements in stars have high gravity, which causes supernovas, which forms nebulas).
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
(As an aside, the heavier elements in stars have high gravity, which causes supernovas, which forms nebulas).
Life Cycle of Stars
Stars have different life cycles, based on their mass.
How does the life cycle of a very heavy star compare to a less-heavy star.
Solution
A heavy star will consume its resources faster in the fusion of hydrogen into helium. Therefore heavy stars have a shorter life span than the less-heavy stars.
Red giants can eventually form black holes.
Solution
Black holes extremely dense bodies formed by extremely heavy stars over 25 times the mass of our Sun.
The Big Bang
Based on the big bang theory, which of the following facts can be deduced?
Solution
The universe was created in a big explosion in which all matter originated from the center of the universe and continues to move outwards from that center.
The universe continues to expand.
All matter is moving outwards in the universe.
The universe continues to expand.
All matter is moving outwards in the universe.
The Big Bang
The universe was created approximately how many years ago?
Solution
Approximately 14 billion (actually ~13.79 billion)
The Big Bang
Which of the following is not evidence for the big bang theory?
Solution
The presence of black holes in the universe is not evidence for the big bang theory
The Big Bang & Absorption Spectrum Information
Edwin Hubble's evidence that the universe is expanding is shown by the absorption spectrum emitted by different galaxies. For galaxies moving away from our galaxy, the absorption spectrum shifts:
Solution
This is absorption spectrum reading is called a red shift and indicates the light is originally from a galaxy that is moving away.
Information has been collected that shows galaxies are moving farther apart from one another and that the universe is expanding.
(Side note: the universe is not just expanding, it is accelerating farther apart).
Information has been collected that shows galaxies are moving farther apart from one another and that the universe is expanding.
(Side note: the universe is not just expanding, it is accelerating farther apart).
Space Technologies
Which technology was developed by a space program?
Solution
All of the above were developed through space programs
Physics: Electricity
Particles in Atoms (Background Review)
Which of the following is located outside of the atom's nucleus?
Solution
Electron orbit the nucleus while protons and neutrons are inside (make up) the nucleus.
What subatomic particle has a positive charge?
Solution
Protons are positive.
Neutrons are neutral.
Electrons are negative
Neutrons are neutral.
Electrons are negative
An atom with 3 protons and 4 electrons will have what charge?
Solution
Protons are positive and electrons are negative. + 3 - 4 = - 1
Which of the following is incorrect?
Solution
Basically everything can change except for the number of protons.
Think about it this way, if scientists could change the number of protons, then they would be able to turn iron into gold!
Think about it this way, if scientists could change the number of protons, then they would be able to turn iron into gold!
Charged Objects
A positively-charged object contains electrons.
Solution
An object is charged when there are more particles of one type than the other type.
A positively charged object has:
(number of positive charges) > (number of negative charges).
E.g.) A positively charged object could have 100 positive charges and 99 electrons.
A positively charged object has:
(number of positive charges) > (number of negative charges).
E.g.) A positively charged object could have 100 positive charges and 99 electrons.
Neutral objects can become positively charged by transferring positive charges from a material.
Solution
Positive charges cannot move. The only way to give a neutral object positive is to take away some of its electrons.
Application of Charges
Laser printers use static charges to print ink onto paper.
Solution
True. The paper is charged, and then the charged ink particles stick to the paper, and it is heated and fixed into place.
Application of Charges
Explain how a powder-coating (paint sprayer) uses static electricity to coat a surface.
Solution
The surface to be painted is attached to an electricity source (of negative electrons). The paint particles are given a positive charge so they are attracted to the surface. The law of electric charges states that opposite charges attract.
The water molecules in humid air can quickly remove the charges on a charged object. Explain what condition is optimal for the powder-coating (paint sprayer) process.
Solution
Dry air...
The Electrostatic Series and Friction
When copper is rubbed against fur, what will happen? Solution
| Attraction of Electrons | Material |
| High attraction | Copper |
| ↕ | Ebonite |
| ↕ | Plastic |
| ↕ | Fur |
| ↕ | Glass |
| Low attraction | Acetate |
Flow of electrons only.
Charge Methods
Which of the following is not a way to charge an object
Solution
Heat
Ground
Describe the flow of charges when a negatively-charged object is brought in contact to a grounded object. [1]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
The grounded object is connected to Earth, which acts as an infinite absorber or source of electrons.
When a negatively-charged object contacts a grounded object, the excess negative electrons are conducted through the grounding into the Earth.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
When a negatively-charged object contacts a grounded object, the excess negative electrons are conducted through the grounding into the Earth.
Insulators and Conductors
Effective and safe transmission of electricity requires the proper use of conductors and insulators.
Materials are insulators when the electrons in atoms are not free to move.
Solution
The transmission of electricity is due to the movement of electrons.
Which of the following is not an insulator?
Solution
Gold is a conductor because its atoms have free electrons that can move and conduct electricity.
Explain why copper wires are wrapped in a plastic coating. [1]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Plastic acts as an insulator to keep the electricity in the metal wire wrapped inside.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Insulators and Conductors
The transmission of electricity requires the presence of free electrons or charged molecules.
Pure (distilled) water acts as a conductor.
Solution
= Insulator.
Salt water (electrolyte) is a conductor.
Solution
The ions in salt water form an electrolyte. Electrolytes can conduct electricity.
Induction
What is induction?
Solution
The charging of an object from proximity without contact
Charging by Induction
Determine the charge remaining on the object, if any.
A positively charged rod is brought close to a neutral, grounded object. While the rod and object are in close proximity, the grounding is removed from the object, then the rod is removed.
Solution
The positively charged rod will attract the negative electrons into the object (positive charges in the ground). When the grounding is removed, the negative charges will be "trapped" in the object.
A positively charged rod is brought close to a neutral, grounded object. The rod is removed from the object, then the grounding is removed from the object, in that order.
Solution
When the rod is removed, since the grounding is still connected to the object, the charges redistribute evenly (neutral).
A negatively charged rod is placed in contact with a neutral, ungrounded object. The rod is removed from the object.
Solution
Placing a charged rod in contact will charge the object.
A negatively charged rod is placed in contact with a neutral, grounded object. The grounding is removed from the object, then the rod is removed from the object, in that order.
Solution
Placing a charged rod in contact will not charge the object when it is grounded. This is because the grounding can be considered as an infinite supply (or sink) of positive and negative charges, which will always make both the rod and the object uncharged (neutral) when connected and grounded.
Lightning
Describe how lightning occurs. [3]
Solution
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Lightning is the result of electric discharge between the bottom of a cloud and the ground. The molecules at the top of a cloud collect positive charges while the molecules at the bottom of a cloud collect negative charges. When the size of the charge imbalance gets high enough, the negative electrons on Earth are repelled leaving a positive charge at the Earth's surface. The negative charge in the cloud and positive charge on the ground cause a release of electrons (electric discharge) from the cloud, through the air as lightning.
Hint
Clear
Info
Incorrect Attempts:
CHECK
Hint Unavailable
Circuit Units
The 3 main quantities in an electrical circuit are resistance, potential difference, and current.
The units of resistance are
Solution
Ohms, Ω
The units of potential difference are
Solution
Volts, V
The units of current are
Solution
Amps, A
Symbols for Resistance, Current, and Potential Difference
What are the correct units to use for resistance, current, and potential difference, respectively?
Solution
- I
- A
- R
- V
- Ω
V, II, and IV
- I
- A
- R
- V
- Ω
Circuit Parts
Electrical energy is gained in _________________, and used in _________________.
Solution
Sources, Loads
Circuit Parts
Which of the following is a load?
Solution
A load converts electrical energy into other forms of energy like light and heat in a lightbulb, or kinetic energy in a motor, or just heat in a resistor.
A Battery
Which of the following statements about a battery is incorrect?
Solution
The electrolyte in a battery creates current.
In a circuit the real particles moving are electrons, which flow from the negative to the positive terminals.
However, due to history, the direction of current is the flow of positive charges (which we know doesn't actually happen).
In a circuit the real particles moving are electrons, which flow from the negative to the positive terminals.
However, due to history, the direction of current is the flow of positive charges (which we know doesn't actually happen).
AC and DC
Electrical generators only produce alternating current (AC).
Solution
Electrical generators do produce alternating current (AC), but they can also produce direct current (DC).
AC and DC
Why is alternating current (AC) typically used to transfer electrical energy long distances from power generation stations to consumers?
Solution
Alternating current (AC) can be transferred in more efficiently
Conventional Current
Which direction does conventional current flow in the circuit:
Solution

Conventional current is the flow of positive charges. The longer side of the cell is positive and the positive charges flow from the positive terminal, to the negative terminal.


Series and Parallel
The diagram represents two...
Solution

light bulbs in parallel
If one of the bulbs goes out or is unplugged from the circuit, then what will happen?
Solution
The other will stay on
Series and Parallel
Adding a load in series to a battery and an original load will...
Solution

The electromotive force (emf) of the source is constant. Potential difference in series is the sum of each load. Adding loads in series will decrease the potential difference in the existing loads. Current is constant for loads in series.
Series and Parallel
Adding a load in parallel would...
Solution
Decrease the total resistance in the circuit... Resistances added in parallel decrease the total resistance.
Potential difference is unchanged in parallel.
Potential difference is unchanged in parallel.
Meters
Different instruments are used to measure current, potential difference, and resistance.
Which of the following measures current only?
Solution
Ammeter... (Multimeters measure current, resistance, and potential difference)
A voltmeter should be connected
Solution
Voltmeters measure voltage.
Parallel paths share the same voltage.
Parallel paths share the same voltage.
An ammeter should be connected
Solution
Ammeters measure current.
Loads on paths in series share the same current.
Loads on paths in series share the same current.
Fuses
A fuse will blow (go out) when which of the following quantities is too high?
Solution
Current is the flow of charges (electrons) over time. If the flow of current is too high, then this could be dangerous and a protective fuse will disconnect the circuit to stop the flow of current.
Electrical Resistance
Resistance in a circuit depends on each of the following except:
Solution
E.g.) each of the following would increase resistance:
A resistive material,
A long wire,
High temperature,
Thin cross-sectional area.
A resistive material,
A long wire,
High temperature,
Thin cross-sectional area.
Electrical Resistance
Which of the following would have the lowest resistance?
Solution
Cold metal that is short and wide (in the path of travel of current)...
Resistance
Three resistors are added in parallel: 2Ω, 3Ω, and 4Ω. Calculate the total resistance.
Solution
RT =
Hint
Clear
Info
Incorrect Attempts:
CHECK
Ω
Hint Unavailable
RT =
Hint
Clear
Info
Incorrect Attempts:
CHECK
Ω
Hint Unavailable
Kirchhoff's Laws
Match the correct equations with the type of circuits below.

- RT = R1 + R2 + ...
- VT = V1 + V2 + ...
- VT = V1 = V2 = ...
- iT = i1 + i2 + ...
- iT = i1 = i2 = ...
A series circuit uses which equations?
Solution
I, III, and VI
A parallel circuit uses which equations?
Solution
II, IV, and V
Kirchhoff's Laws
Complete the following using the laws of Gustav R. Kirchhoff (1824 - 1887).
Determine the voltage across resistor 1.
Solution

The total increase in electric potential (voltage) is equal to the total decrease in electric potential across one path in a circuit.
 VT = V1 + V2
VT = V1 + V2
 VT = V1 + V2
VT = V1 + V2
Determine the voltage across resistor 2.
Solution

The total increase in electric potential (voltage) is equal to the total decrease in electric potential across one path in a circuit.
 VT = V1 = V2
VT = V1 = V2
 VT = V1 = V2
VT = V1 = V2
Determine the current across resistor 1.
Solution

The current at the start of a junction is equal to the current at the end point of that junction.
 i0 = i1 = i2
i0 = i1 = i2
 i0 = i1 = i2
i0 = i1 = i2
Determine the current across resistor 2.
Solution

The current at the start of a junction is equal to the current at the end point of that junction.
 i0 = i1 + i2
i0 = i1 + i2
 i0 = i1 + i2
i0 = i1 + i2
Calculations with V = IR
Answer the questions using the relationship with potential difference (V), current (I), and resistance (R) below.
V = I · RWhen the electromotive force (emf) in a circuit is increased with a constant resistor, the current increases.
Solution
Electromotive force (emf) is the volts in the source, like a battery. If V increases and R stays the same, then this means I increases.
A circuit has one resistor connected to a battery with a 5.0 V emf and a 0.5 A current. Calculate the value of the resistor.
Solution
A new circuit has two 4.0 Ω resistors connected in series, shown below. If the total electromotive force in the circuit is 8.0 V, calculate the current in each resistor.
Solution
 I =
Hint
Clear
Info
Incorrect Attempts:
CHECK
A
Hint Unavailable
For resistors in series, current is the same in each.
I =
Hint
Clear
Info
Incorrect Attempts:
CHECK
A
Hint Unavailable
For resistors in series, current is the same in each.
Calculate the current (I) in just one of the resistors:
Therefore the current in each resistor is 2 Amps.
I =
Hint
Clear
Info
Incorrect Attempts:
CHECK
A
Hint Unavailable
Calculate the current (I) in just one of the resistors: Therefore the current in each resistor is 2 Amps.
Electrical Calculations
A 2 Amp motor is run over 3 minutes and has a total resistance of 10Ω. Solve for the total Energy, given the equations below.
Solution
V = I · R
P = I · V
E = P · t
E =
Hint
Clear
Info
Incorrect Attempts:
CHECK
J
Hint Unavailable
Current = I = Amps = A
Resistance = R = Ohms = Ω
Voltage = Volts = V
Energy = Joules = J
Power = Watts = W
(Time is in seconds)
First calculate the voltage, V (potential difference):
Second calculate the power, P:
Third calculate the energy, E:
E =
Hint
Clear
Info
Incorrect Attempts:
CHECK
J
Hint Unavailable
Resistance = R = Ohms = Ω
Voltage = Volts = V
Energy = Joules = J
Power = Watts = W
(Time is in seconds) First calculate the voltage, V (potential difference): Second calculate the power, P: Third calculate the energy, E:
Electricity Generation
All of the following are commonly used to generate electricity except which one?
Solution
Sound
Electricity Generation
Which one is a non-renewable energy resource?
Solution
Nuclear
Power
The units of power are
Solution
Watts, W
Power
When electricity is consumed, it is often called "power". The units for power are kilowatt·hour
kW·hOne kilowatt·hour equals one kilowatt multiplied by one hour.
Solution
True
How many watts (W) are in 1 kilowatt (kW)?
Solution
1 kilowatt (kW) = 1000 watts (W)
Calculate the efficiency of a dishwasher if it uses 2000 kW·h when a total of 2600 kW·h is input.
Solution
If the price of electricity is 10 ¢/kW·h, calculate the cost to run a 1000 W appliance for an hour and a half.
Solution
Cost = Rate × Power Use
It should make sense that the cost is the rate times the power usage in kW·h
Rate = 10 ¢/kW·h
! 1000 W = 1 kW
Power Use = (1 kW)(1.5 hr) = 1.5 kW·h
Rate = 10 ¢/kW·h
! 1000 W = 1 kW
Power Use = (1 kW)(1.5 hr) = 1.5 kW·h
Scientific Investigation and Exploration
The Relationship Between Scientific Ideas, Hypothesis, and Data
Which of the following would be an effective way to help show the occurrence of global warming?
Solution
A good study will use the data over many years like the graph of average of the daily temperature data around the world for the past 100 years. This is a visual representation of the numeric data collected over as long a time span as possible for reliable data collection. The more data the better. Also a combination of many of the other data collections would be useful.
Formulating a Hypothesis Vs. Facts and Issues
Which of the following would be a hypothesis for the issue regarding: the use of solar and wind energy, instead of burning fossil fuels (oil, gas, etc.)?
Solution
A hypothesis is a proposition or explanation made without knowing the truth of it yet. This is posed as a statement (rather than as a question) and forms the basis for the research or questions that will be the scientific investigation to follow.
Selecting Appropriate Equipment and Materials for Scientific Inquires
A student wishes to measure the effects of acid rain on their local pond near a field. What item would be most necessary to include in this student's toolkit in the field?
Solution
The student must first check if the pond is acidic or not. This is done with pH paper.
Workplace Hazardous Materials Information System (WHMIS)
Which of the following is WHMIS not used for?
Solution
The WHMIS book contains information on:
- Product information, including manufacturer and contact information
- Hazards, toxicology, and safety data
- Physical properties
- Etc...
- Product information, including manufacturer and contact information
- Hazards, toxicology, and safety data
- Physical properties
- Etc...
Appropriate Handling and Disposal Techniques
At the end of a classroom experiment in which several different chemicals were combined into a cool bubbly-foamy reaction, there are some extra chemicals left over. What should be done with these extra leftover chemicals?
Solution
As a general guideline,
- Don't put unused chemicals back into their original container. (Unless specifically instructed to do so by your teacher.)
- Don't pour chemicals down the sink drain. (Unless specifically instructed to do so by your teacher.)
- Don't put chemicals in the garbage. (Unless specifically instructed to do so by your teacher.)
- Don't put unused chemicals back into their original container. (Unless specifically instructed to do so by your teacher.)
- Don't pour chemicals down the sink drain. (Unless specifically instructed to do so by your teacher.)
- Don't put chemicals in the garbage. (Unless specifically instructed to do so by your teacher.)
Safety Equipment
Which of the following pieces of safety equipment is not considered necessary?
Solution
Be careful! Always wear all your safety stuff in the chemistry lab! Gloves, Goggles, Protective clothing and shoes are the main protective methods in a lab. Sometimes even more protection is used depending on the experiment.
Gathering Data in a Lab
A student wants to compare how higher temperature allows more salt to dissolve in water. To record and organize the temperature and amount of salt properly, the student should Solution
_onder_de_microscoop_creativeCommons.jpg) |
It is important to record more than one data point for each test, then these can be averaged to reduce error.
Controlling Variables
A controlled experiment changes two or more variables at a time to determine a change in a dependent variable.
Solution
A controlled experiment only changes one independent variable at a time, and measures the change or effect on the dependent variable.
Dependent and Independent Variables
A dependent variable is one which
Solution
A dependent variable changes due to the change in another variable, which is known as the independent variable. For example time is the independent variable and concentration of a chemical is the dependent variable. On an x-y graph, the 'y' values is dependent on the 'x' value.
Qualitative and Quantitative Data
Which of the following is not a quantitative observation?
Solution
Qualitative Observations
Qualitative observations are based on descriptions from sensory information like: sight, smell, texture, colour, values like good or bad, etc.
Quantitative Observations
Quantitative observations are based on numerical data that can be measured like: length, weight, volume, time, area, speed, temperature, number, etc...
Qualitative observations are based on descriptions from sensory information like: sight, smell, texture, colour, values like good or bad, etc.
Quantitative Observations
Quantitative observations are based on numerical data that can be measured like: length, weight, volume, time, area, speed, temperature, number, etc...
